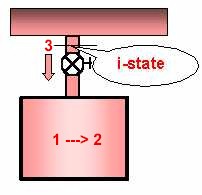
In this discharge problem the uniform open
system, which can be represented by a single state at any given instant,
executes an open process as it goes from a clearly defined b-State to a unique
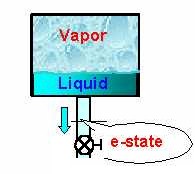
f-State. During the process the discharge at e-State remains steady.
We first evaluate the three states (
State-1, 2 and 3) as much as possible
from the given data, do a process analysis, and then update
all calculations with the help of the Super-Cal button.
Because the problem is in English system,
click on the English radio button.
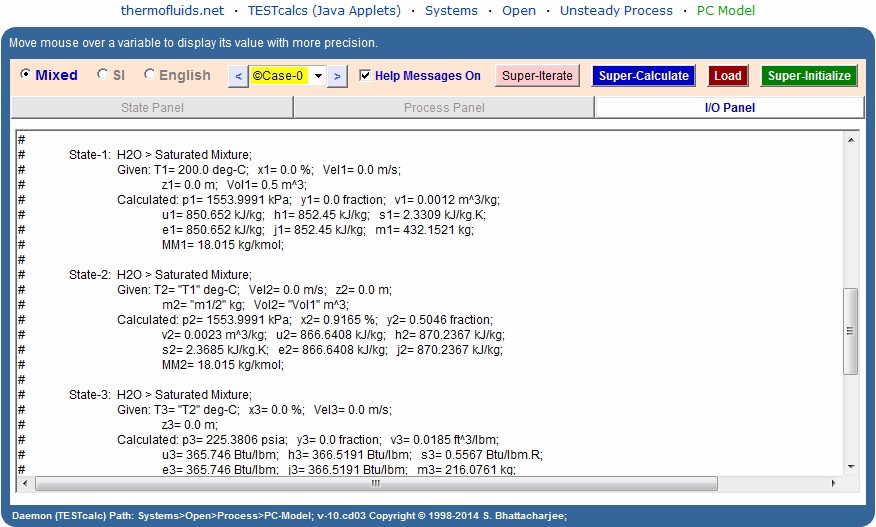
State-1: Enter p1, y1 (volume fraction is 50%), Vol1, and
Calculate. The mass is calculated as 5.841 lbm.
State-2: Enter p2 ('=p1' maintained by the valve), x2 (100%),
Vol2 ('=Vol1'), and Calculate. The mass is calculated as 0.019 lbm.
State-3: Enter p3 ('=p1'), x3 (=100% as saturated vapor is
being released), and Calculate.
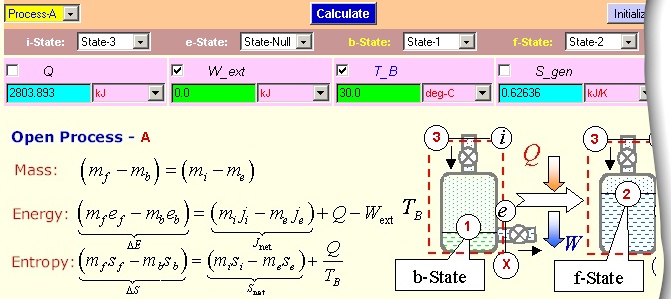
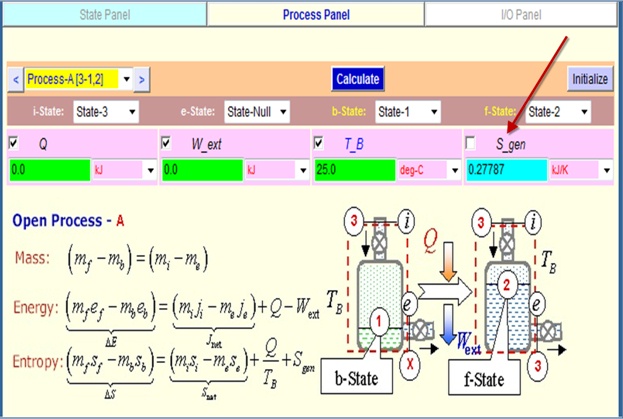
On the Analysis panel, load State-1 as the b-State, State-2 as the f-State
and State-3 as the e-State. Enter the known process variable
W_ext (=0) and T_B (= 300o C). A Calculate and Super-Cal produce
m_3=5.82 lbm .
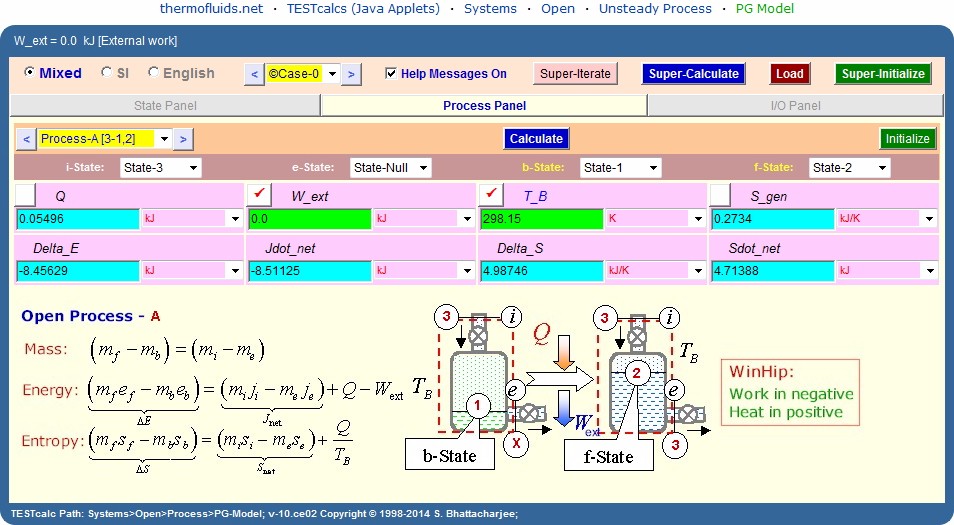
For the what-if study, go to States
panel, choose State-1
and change p1 to 60 psia. A Calculate
and Super-Cal produce m_e=m_3=5.74
lbm and Q=5270 Btu.
Note the negative value of S_gen which results
because of the choice of the default value of T_B. If T_B is changed to
a realistic value, say the flame temperature responsible for the heating,
S_gen will become positive. A value of T_B=2000F produces (in the second
part of the problem) a S_gen=1 Btu/R.
|